In general, curcumin studies have demonstrated that dietary administration of the compound in doses up to 12 g per day is well tolerated; however, its effects on slowing or reversing cognitive decline have been modest at best and very often dependent on the stage of AD when treatment commences. For example, in an Asian study of 1,010 non-demented individuals, a small but statistically significant improvement in cognitive abilities was noted in a population that consumed curry more than once per month. By contrast, in a more recent six-month randomized study, patients with moderateto-severe Alzheimer’s disease showed little or no measureable improvement when compared with placebo controls. These clinical findings conflict with data obtained from curcumin-treated animal models and suggest challenges lie ahead in translating findings from rodent studies to human trials. Perhaps these challenges can be met by more clearly defining the objective of curcumin treatment; either as a preventative to delay or avert the onset of significant cognitive impairment in early stage AD patients or as a therapeutic aimed at reversing the clinical hallmarks of dementia found in more advanced stages. Thus far, the majority of rodent studies have been carried out by Silmitasertib administering curcumin to animals prior to their developing AD pathologies, whereas the majority of human trials that have been attempted largely recruit individuals who are already symptomatic of AD and likely to have significant amyloid plaque burden. Reversing an already substantial plaque load may require multiple therapeutic modalities to supplement curcumin’s bioactivity or, alternatively, a more effective compound targeting Ab plaque development such as the improved inhibitor 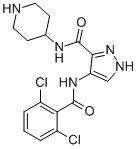 presented here. Tuberculosis is still a worldwide problem as the number of new cases continues to grow, approaching 9.8 million in 2010 and resulting in approximately 1.68 million deaths in 2009. Human immunodeficiency virus co-infection is a crucial factor in the rise in the number of TB cases and the development of active tuberculosis. In addition, multidrug resistant and extensively drug resistant strains continue to evolve, making current treatments ineffective. To counter the drug resistance problem there is a crucial need to identify new drug targets. Inosine monophosphate is obtained in mycobacteria by the de novo purine nucleotide biosynthesis pathway wherein the purine ring is assembled in a stepwise manner starting from phosphoribosyl pyrophosphate through eleven distinct enzymatic steps. IMP is a common precursor for both adenine and guanine nucleotide synthesis. The first of the two steps towards guanine nucleotide biosynthesis is CHIR-99021 252917-06-9 catalysed by inosine monophosphate dehydrogenase which converts IMP to xanthosine monophosphate with the concomitant conversion of NAD+ to NADH. The IMPDH reaction equilibrium strongly favors the forward reaction and maintains the guanine nucleotide pool. In M. tuberculosis Mt-GuaB2 is solely responsible for this essential function, since out of the three genes that encode IMPDH Mt-GuaB2 is the only functional ortholog. IMPDH is considered an attractive target for immunosuppressive, cancer, antiviral, and antimicrobial therapy. A genome wide transposon mutagenesis study indicated that M. tuberculosis requires Mt-GuaB2 for its survival. IMPDH inhibitors cause a reduction of guanine nucleotide levels and increase adenine nucleotides in vivo, and subsequently, DNA and RNA synthesis is interrupted resulting in cytotoxicity. Depending on the mode of enzyme binding, IMPDH inhibitors are classified into three types: type I inhibitors are IMP/XMP analogues, type II are NADH analogues and type III are multisubstrate inhibitors.
presented here. Tuberculosis is still a worldwide problem as the number of new cases continues to grow, approaching 9.8 million in 2010 and resulting in approximately 1.68 million deaths in 2009. Human immunodeficiency virus co-infection is a crucial factor in the rise in the number of TB cases and the development of active tuberculosis. In addition, multidrug resistant and extensively drug resistant strains continue to evolve, making current treatments ineffective. To counter the drug resistance problem there is a crucial need to identify new drug targets. Inosine monophosphate is obtained in mycobacteria by the de novo purine nucleotide biosynthesis pathway wherein the purine ring is assembled in a stepwise manner starting from phosphoribosyl pyrophosphate through eleven distinct enzymatic steps. IMP is a common precursor for both adenine and guanine nucleotide synthesis. The first of the two steps towards guanine nucleotide biosynthesis is CHIR-99021 252917-06-9 catalysed by inosine monophosphate dehydrogenase which converts IMP to xanthosine monophosphate with the concomitant conversion of NAD+ to NADH. The IMPDH reaction equilibrium strongly favors the forward reaction and maintains the guanine nucleotide pool. In M. tuberculosis Mt-GuaB2 is solely responsible for this essential function, since out of the three genes that encode IMPDH Mt-GuaB2 is the only functional ortholog. IMPDH is considered an attractive target for immunosuppressive, cancer, antiviral, and antimicrobial therapy. A genome wide transposon mutagenesis study indicated that M. tuberculosis requires Mt-GuaB2 for its survival. IMPDH inhibitors cause a reduction of guanine nucleotide levels and increase adenine nucleotides in vivo, and subsequently, DNA and RNA synthesis is interrupted resulting in cytotoxicity. Depending on the mode of enzyme binding, IMPDH inhibitors are classified into three types: type I inhibitors are IMP/XMP analogues, type II are NADH analogues and type III are multisubstrate inhibitors.