The antiangiogenic effects were attributed to BAY-60-7550 decreased production of VEGF and resistance of endothelial cells to VEGF stimulation. Further studies used the rapalog RAD001 and compared its effects with known antiangiogenic agents. The results showed that RAD001 was found to be associated with decreasing the tumor vessel density and the maturity of the tumor vessels, whereas the antiangiogenic 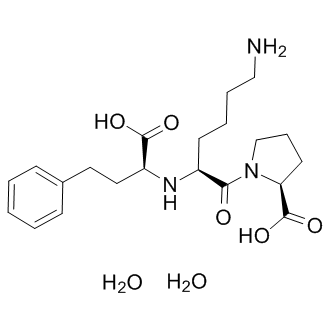 drug vatalanib was found to impact only the microvascular density but not the vessel maturity consistent with this class of drugs which impact the VEGF/ VEGFR complex. On the other hand, Zhang et al. reported that the aSMA level, a marker of mature pericytes, increased in Tubulin Acetylation Inducer rapamycin treated tumor compared with non-treated tumor. Other studies have shown that radiation induces activation of mTOR pathways in the tumor endothelial cells making them more sensitive to response with rapamycin. However, a more recent study using a retro-inhibition approach found that HNSCC cells and not the tumor microenvironment as the target for rapamycin activity and that the anti-angiogenic effect is a likely downstream consequence of mTOR inhibition in cancer cells. Imaging of properties intrinsic to tumor physiology such as tumor pO2 and tumor microvessel density made it possible to sequentially follow rapamycin induced changes during the course of treatment non-invasively and sequentially during the treatment course. The key finding in the present study pertaining to the rapamycin effect on tumor physiology is that the tumor microvessel density, when monitored longitudinally showed a significant decrease whereas a transient increase in tumor pO2 was found followed by onset of hypoxia. It should be noted that the USPIO-based blood volume assessment may overestimate the values in tumors because of their leakiness compared to normal tissues. The observations from histological experiments were in agreement with the imaging observations. The rapamycin-induced decrease in CD31 staining was found to be in agreement with imaging experiments where a loss in microvessel density was found. However, there was a small but non-significant decrease in staining of aSMA which reflects the retention of the integrity of the pericyte coverage of the tumor vasculature after rapamycin administration. These results indicate that rapamycin treatment pruned immature blood vessels rather than mature blood vessels. It is expected that these changes in tumor microvasculature can cause improvement of blood flow, a phenomenon known as vascular normalization. The transient increase in the pO2 by rapamycin treatment can be attributed to the increased blood flow in the tumor, which was demonstrated by a 40% increase in tumor initial uptake of Gd-DTPA 2 days after rapamycin treatment in the DCE-MRI study. The identification of transient improvements in tumor oxygenation 2 days after rapamycin treatment provides an opportunity for chemoradiation modalities where radiation therapy can be timed to take advantage of increases in tumor pO2 to elicit improved response. The results in the present study show enhancement in tumor radioresponse by rapamycin treatment. This data suggests that the transiently increased level of median tumor pO2 in rapamycin treated mice compared to the day matched control group may be responsible for the observed effect of radioresponse with combination treatment.
drug vatalanib was found to impact only the microvascular density but not the vessel maturity consistent with this class of drugs which impact the VEGF/ VEGFR complex. On the other hand, Zhang et al. reported that the aSMA level, a marker of mature pericytes, increased in Tubulin Acetylation Inducer rapamycin treated tumor compared with non-treated tumor. Other studies have shown that radiation induces activation of mTOR pathways in the tumor endothelial cells making them more sensitive to response with rapamycin. However, a more recent study using a retro-inhibition approach found that HNSCC cells and not the tumor microenvironment as the target for rapamycin activity and that the anti-angiogenic effect is a likely downstream consequence of mTOR inhibition in cancer cells. Imaging of properties intrinsic to tumor physiology such as tumor pO2 and tumor microvessel density made it possible to sequentially follow rapamycin induced changes during the course of treatment non-invasively and sequentially during the treatment course. The key finding in the present study pertaining to the rapamycin effect on tumor physiology is that the tumor microvessel density, when monitored longitudinally showed a significant decrease whereas a transient increase in tumor pO2 was found followed by onset of hypoxia. It should be noted that the USPIO-based blood volume assessment may overestimate the values in tumors because of their leakiness compared to normal tissues. The observations from histological experiments were in agreement with the imaging observations. The rapamycin-induced decrease in CD31 staining was found to be in agreement with imaging experiments where a loss in microvessel density was found. However, there was a small but non-significant decrease in staining of aSMA which reflects the retention of the integrity of the pericyte coverage of the tumor vasculature after rapamycin administration. These results indicate that rapamycin treatment pruned immature blood vessels rather than mature blood vessels. It is expected that these changes in tumor microvasculature can cause improvement of blood flow, a phenomenon known as vascular normalization. The transient increase in the pO2 by rapamycin treatment can be attributed to the increased blood flow in the tumor, which was demonstrated by a 40% increase in tumor initial uptake of Gd-DTPA 2 days after rapamycin treatment in the DCE-MRI study. The identification of transient improvements in tumor oxygenation 2 days after rapamycin treatment provides an opportunity for chemoradiation modalities where radiation therapy can be timed to take advantage of increases in tumor pO2 to elicit improved response. The results in the present study show enhancement in tumor radioresponse by rapamycin treatment. This data suggests that the transiently increased level of median tumor pO2 in rapamycin treated mice compared to the day matched control group may be responsible for the observed effect of radioresponse with combination treatment.