Thus, the increase in SOD observed in the hepatic tissue is beneficial, because it suggests an adequate response to this superoxide excess. Conversely, treatment with U. tomentosa resulted in a reduction of the SOD levels in the tumour 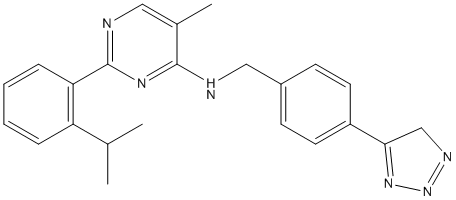 tissue, which in turn constitutes an advantageous result as well, as it indicates that the tumour cells are being left more susceptible to the effects of the superoxide anion. This apparent selectivity was also observed for Cat levels. The importance of Cat in the neoplastic scenarios has long been appreciated, as various studies have demonstrated that increased activity of this enzyme in tumour cells constitute a protection mechanism against cell death induced by reactive oxygen species, and that by inhibiting this enzyme, the neoplastic cells become once more vulnerable to this outcome. Our findings indicate that Cat levels were reduced in the tumour tissue and increased in the liver, both these results occurring simultaneously and due to the treatment with the U. tomentosa BHE extract. Indeed, we may be witnessing a protective effect on the liver associated with a further exposure of the tumour cells to ROS-induced necrosis/apoptosis. Meaning to further characterize this observation, the protein expressions of both catalase and SOD-1 were assessed by Western blot. As no statistically significant differences were found among the experimental groups, it seems appropriate to assume that the treatments could be increasing the individual output of these enzymes, resulting in a greater overall activity despite equivalent expression rates. In addition to this hypothesis, the lack of differences in the expression of SOD-1 does not exclude an eventual effect upon the expression of the other two isoforms of this enzyme, namely SOD2 and SOD3. According to Valko et al., the lower Cat activity induced by various tumours has been attributed to an increase in TNF-a level, which reduces hepatic Cat activity. The BHE extract and its BuOH fraction expressively reduced the TNF-a level on the liver homogenates of tumour-bearing rats when compared to control, perhaps contributing to a recovery of the hepatic Cat activity in these animals. The mensuration of this cytokine in liver homogenates also indicates an anti-inflammatory activity of both the BHE extract and its BuOH fraction. Interestingly, however, we observed an effective reversal of TNF-a to baseline values as a result of the former treatment, leading us to conclude that, regarding this parameter, the BHE extract is MK-2206 Akt inhibitor indeed more effective than its BuOH fraction. The CHCl3 fraction exhibited a mild reduction of this cytokine, which seems natural considering the recognized anti-inflammatory properties of isolated pentacyclic FDA-approved Compound Library oxindole alkaloids. On the whole, reduction of the cytokine TNF-a is bound to ameliorate some hallmarks of this tumour, such as cachexia. The tumour levels of TNF-a were quite lower when compared to those of the liver. This is probably caused by the histological differences between these tissues; with the latter containing TNF-a-secreting Kupffer cells and the former encompassing mainly fibroblasts that do not secrete TNF-a. Regarding the tumour production of this cytokine, while the same tendencies as those of the liver were observed, no statistical significances were found.
tissue, which in turn constitutes an advantageous result as well, as it indicates that the tumour cells are being left more susceptible to the effects of the superoxide anion. This apparent selectivity was also observed for Cat levels. The importance of Cat in the neoplastic scenarios has long been appreciated, as various studies have demonstrated that increased activity of this enzyme in tumour cells constitute a protection mechanism against cell death induced by reactive oxygen species, and that by inhibiting this enzyme, the neoplastic cells become once more vulnerable to this outcome. Our findings indicate that Cat levels were reduced in the tumour tissue and increased in the liver, both these results occurring simultaneously and due to the treatment with the U. tomentosa BHE extract. Indeed, we may be witnessing a protective effect on the liver associated with a further exposure of the tumour cells to ROS-induced necrosis/apoptosis. Meaning to further characterize this observation, the protein expressions of both catalase and SOD-1 were assessed by Western blot. As no statistically significant differences were found among the experimental groups, it seems appropriate to assume that the treatments could be increasing the individual output of these enzymes, resulting in a greater overall activity despite equivalent expression rates. In addition to this hypothesis, the lack of differences in the expression of SOD-1 does not exclude an eventual effect upon the expression of the other two isoforms of this enzyme, namely SOD2 and SOD3. According to Valko et al., the lower Cat activity induced by various tumours has been attributed to an increase in TNF-a level, which reduces hepatic Cat activity. The BHE extract and its BuOH fraction expressively reduced the TNF-a level on the liver homogenates of tumour-bearing rats when compared to control, perhaps contributing to a recovery of the hepatic Cat activity in these animals. The mensuration of this cytokine in liver homogenates also indicates an anti-inflammatory activity of both the BHE extract and its BuOH fraction. Interestingly, however, we observed an effective reversal of TNF-a to baseline values as a result of the former treatment, leading us to conclude that, regarding this parameter, the BHE extract is MK-2206 Akt inhibitor indeed more effective than its BuOH fraction. The CHCl3 fraction exhibited a mild reduction of this cytokine, which seems natural considering the recognized anti-inflammatory properties of isolated pentacyclic FDA-approved Compound Library oxindole alkaloids. On the whole, reduction of the cytokine TNF-a is bound to ameliorate some hallmarks of this tumour, such as cachexia. The tumour levels of TNF-a were quite lower when compared to those of the liver. This is probably caused by the histological differences between these tissues; with the latter containing TNF-a-secreting Kupffer cells and the former encompassing mainly fibroblasts that do not secrete TNF-a. Regarding the tumour production of this cytokine, while the same tendencies as those of the liver were observed, no statistical significances were found.