Moreover, small chemical compounds fostering aggregation of VP26 might be developed into effective 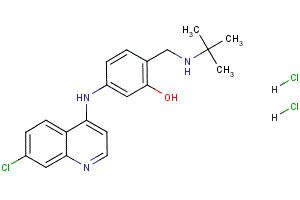 antiviral therapy that prevents HSV nuclear capsid egress and thus virion formation. On the other hand, for characterization of intracellular capsid trafficking, virion assembly and cell entry, we will base future tagging or disabling mutations in HSV1 proteins on HSV1 that has a low propensity for nuclear aggregation, and therefore seems to contain a less invasive tag on VP26. Furthermore, its subcellular capsid distribution during the course of an infection resembles more that of untagged capsids when compared to the other tags on VP26. Her-2, Estrogen Mepiroxol receptor and Progesterone Receptor are the most commonly used biomarkers and therapeutic targets in breast cancer patients. However, these biomarkers are not expressed in 17�C30% of women with breast cancer which limits the use of existing therapies. Patients under hormone deprivation and Herceptin therapy, a most common therapeutic option, tend to acquire resistance to such therapies over time. Whereas, the triple negative breast cancer phenotype, which lacks the presence of Her-2, ER and PR are even more aggressive and resistant. Therefore there is an urgent clinical need to identify new diagnostic as well as therapeutic markers for early diagnosis and treatment of such patients. Herceptin, like other humanized receptor targeted monoclonal antibodies, inhibits the growth and progression in Her-2 positive breast tumors by blockade of downstream survival pathway. However, recent reports suggest that cells acquire resistance to the targeted therapies against receptor tyrosine kinases by several mechanisms. One of the most commonly seen mechanism is the activation of other receptor RTKs such as EGFR, IGFR and non-receptor tyrosine kinases such Src. The overexpression of EGFR and Src in both Her-2 negative and TNBC cells contributes significantly to the tumor growth and progression. Considering the heterogeneity of cancer cells, it is predicted that not only these RTKs, but also other proteins which are required for normal functioning of these proteins are also upregulated in such cells. We found that Annexin A2, a calcium dependent phospholipid binding protein, is inversely correlated with Her-2 expression. This observation holds true in case of Herceptin resistance, both in experimental and clinical situations. AnxA2 is aberrantly expressed in various human cancers. It is present as a monomer in the nucleus, but as a heterotetramer with p11 in the cytosol to bind to the inner and outer leaflets of the plasma membrane. The cytosolic AnxA2 is mobilized to the cell Atropine sulfate surface upon phosphorylation at the Nterminal Serine 25 and Tyrosine 23, by different kinases such as PKC and Src as well as treatment with calcium ionophore or calcium inducing agents such as glutamate. The cell surface associated AnxA2 heterotetramer, is a receptor for both plasminogen and tissue type plasminogen activator and acts as a catalytic center for the activation of plasminogen to plasmin which helps in invasion and metastasis of cancer cells. The membrane associated AnxA2 interacts with RTKs such as like insulin receptor, insulin-like growth factor receptor and non-receptor tyrosine kinases such as focal adhesion kinase and Src. AnxA2 acts as a key scaffolding protein in anchoring and transportation of several proteins within plasma membrane as well as from cytosol to the plasma membrane, and contributes to cell signaling, angiogenesis and matrix degeneration. Our recent data show that stimulation of AnxA2 by calcium ionophore or a phosphomimetic mutant of AnxA2 leads to its localization to the lipid raft component of the cell membrane, where it interact with different proteins and also leads to its own exosomal association.
antiviral therapy that prevents HSV nuclear capsid egress and thus virion formation. On the other hand, for characterization of intracellular capsid trafficking, virion assembly and cell entry, we will base future tagging or disabling mutations in HSV1 proteins on HSV1 that has a low propensity for nuclear aggregation, and therefore seems to contain a less invasive tag on VP26. Furthermore, its subcellular capsid distribution during the course of an infection resembles more that of untagged capsids when compared to the other tags on VP26. Her-2, Estrogen Mepiroxol receptor and Progesterone Receptor are the most commonly used biomarkers and therapeutic targets in breast cancer patients. However, these biomarkers are not expressed in 17�C30% of women with breast cancer which limits the use of existing therapies. Patients under hormone deprivation and Herceptin therapy, a most common therapeutic option, tend to acquire resistance to such therapies over time. Whereas, the triple negative breast cancer phenotype, which lacks the presence of Her-2, ER and PR are even more aggressive and resistant. Therefore there is an urgent clinical need to identify new diagnostic as well as therapeutic markers for early diagnosis and treatment of such patients. Herceptin, like other humanized receptor targeted monoclonal antibodies, inhibits the growth and progression in Her-2 positive breast tumors by blockade of downstream survival pathway. However, recent reports suggest that cells acquire resistance to the targeted therapies against receptor tyrosine kinases by several mechanisms. One of the most commonly seen mechanism is the activation of other receptor RTKs such as EGFR, IGFR and non-receptor tyrosine kinases such Src. The overexpression of EGFR and Src in both Her-2 negative and TNBC cells contributes significantly to the tumor growth and progression. Considering the heterogeneity of cancer cells, it is predicted that not only these RTKs, but also other proteins which are required for normal functioning of these proteins are also upregulated in such cells. We found that Annexin A2, a calcium dependent phospholipid binding protein, is inversely correlated with Her-2 expression. This observation holds true in case of Herceptin resistance, both in experimental and clinical situations. AnxA2 is aberrantly expressed in various human cancers. It is present as a monomer in the nucleus, but as a heterotetramer with p11 in the cytosol to bind to the inner and outer leaflets of the plasma membrane. The cytosolic AnxA2 is mobilized to the cell Atropine sulfate surface upon phosphorylation at the Nterminal Serine 25 and Tyrosine 23, by different kinases such as PKC and Src as well as treatment with calcium ionophore or calcium inducing agents such as glutamate. The cell surface associated AnxA2 heterotetramer, is a receptor for both plasminogen and tissue type plasminogen activator and acts as a catalytic center for the activation of plasminogen to plasmin which helps in invasion and metastasis of cancer cells. The membrane associated AnxA2 interacts with RTKs such as like insulin receptor, insulin-like growth factor receptor and non-receptor tyrosine kinases such as focal adhesion kinase and Src. AnxA2 acts as a key scaffolding protein in anchoring and transportation of several proteins within plasma membrane as well as from cytosol to the plasma membrane, and contributes to cell signaling, angiogenesis and matrix degeneration. Our recent data show that stimulation of AnxA2 by calcium ionophore or a phosphomimetic mutant of AnxA2 leads to its localization to the lipid raft component of the cell membrane, where it interact with different proteins and also leads to its own exosomal association.
Month: June 2019
With BMU control the challenge of understanding bone volume homeostasis is clearly daunting
The issue confronting us is how to make sense of available data on BMUs, and to turn this data into an integrated understanding of bone physiology that has explanatory power. This is typically the role of quantitative or theoretical models. There has been a fairly long history of mathematical and computational models of events in bone turnover. Earlier models, such as those summarized in Martin et al. tended to focus on questions of rates of bone turnover e.g. what rate of resorption, mineralization, or BMU activation. More recently, computational models have been developed to model the evolution of various bone cell lineages and the role of specific signaling molecules. Spatial aspects of cell organization within trabecular and Gomisin-D cortical BMUs have very recently also been considered. These past models tend to be based on systems of differential equations. A somewhat different approach to these past bone models is to look to ‘Albaspidin-AA control theory’ to provide some kind of framework for interpreting the available information on a BMU, as it deals with principles of control of dynamical systems. Indeed, that is what we will attempt to do in this paper and is the general conceptual approach that has been taken by others to understand bone regulation. By doing this we hope to ‘step back’ from specific processes/interactions in a bone remodeling event and instead focus on the general requirements to achieve bone balance as well as the constraints this then imposes on various interactions. This should help to provide an explanation for observations in terms of control of bone balance and to systematically predict other currently unknown control mechanisms. In contrast, previous bone models of specific processes/ interactions can be viewed as specific examples of these constraints known or assumed to occur. The classical design control issue is ensuring a system maintains a constant single output for a single input. This is typically achieved using negative feedback control, so that the input is adjusted to achieve a desired output. However, more complex systems with multiple processes also require control mechanisms to see that separate processes within the system are coordinated. Therefore within a BMU, we may expect to see negative feedback control and process coordination control. Both mechanisms need to be considered for the homeostatic control of bone volume by a single BMU. To make any progress, it is clear we first need to reduce the complexity described above. To do this, we would like the BMU to be operating in as simple a way as possible. So for the purposes of this study, we first assume that the BMU is established and steadily moving through the cortical bone. We further assume that signals from the ‘whole body’ level and from the ‘regional’ level to the BMU are in an averaged sense, time invariant. This enables us to focus on fundamental bone balance mechanisms operating within the BMU itself. This situation might be approximated in a young, healthy adult, with constant bone volume and normal bone turnover. Even with these simplifications, there remain many signaling systems operating within the BMU, and no one is sure how the actions of these signaling molecules are integrated to maintain bone balance. To tackle this problem one may first ask: what needs to happen in a BMU to ensure bone balance is maintained? What are the different possible general ways that bone balance may be achieved? Having answered these questions one 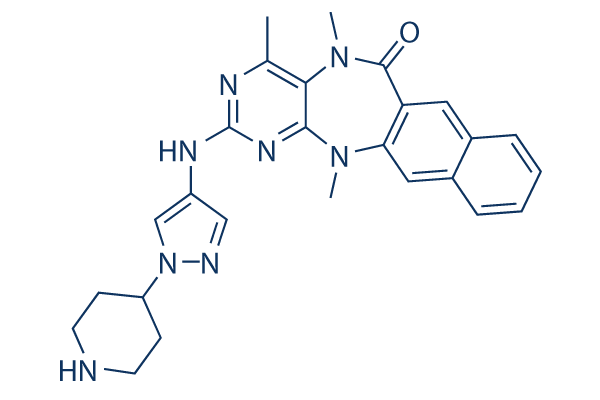 may then ask: what signaling processes and mechanisms within the BMU are potentially part of the BMU control systems to maintain bone balance?
may then ask: what signaling processes and mechanisms within the BMU are potentially part of the BMU control systems to maintain bone balance?
It also has a transcriptional repressor domain that is important for the development of melanocytes
While Pax3 acts as a transcriptional activator to promote myogenesis. It will be of interest to determine whether Pax3 inhibits chondrogenesis by acting as a transcriptional repressor or activator in the satellite cells. It will be also of interest to investigate whether other myogenic factors play inhibitory roles in Atropine sulfate chondrogenic differentiation. We also discovered a novel function for Sox9 in this study. Sox9 is the master regulator of chondrogenesis, as no cartilage formation takes place in the absence of Sox9. Sox9 acts as a transcriptional activator in chondrogenic precursor cells by binding to the Tulathromycin B promoters of cartilage-specific matrix genes collagen II and aggrecan. We found that Sox9 strongly induced collagen II and aggrecan expression, as well as glycosaminoglycan level in the muscle satellite cells, which normally are non-chondrogenic precursors, consistent with its activity in the somite. In the meantime, Sox9 also significantly, although weakly, inhibited the expression of early muscle lineage marker Pax3 and Pax7, as well as myosin heavy chain. It has been reported that Sox9 is expressed in the satellite cells, and has the ability to inhibit a-sarcoglycan expression in the C2C12 myoblast cell line and the myogenin promoter in 10T1/2 cells. Our data are consistent with these reports. While Sox9 may be expressed in satellite cells, it is apparent from our work and others that Sox9 is much more strongly expressed in chondrocytes, and that ectopic expression of Sox9 leads to chondrogenic differentiation and maintenance of the chondrocyte phenotype. Our data suggest that Nkx3.2 plays a central role in the chondrogenic differentiation of satellite cells, and that its activity is required for Sox9 to promote chondrogenesis and inhibit myogenesis. Like Sox9, Nkx3.2 is expressed in the cartilage precursors in the embryo, and promotes cartilage cell fate in the somites. Nkx3.2 null mice exhibit reduced cartilage formation including a downregulation of Sox9 expression.  Inactivating mutations of Nkx3.2 in human lead to spondylo-megaepiphyseal-metaphyseal dysplasia, a disease that causes abnormalities of the vertebral bodies, limbs and joints. Here we show that Nkx3.2 is activated in the muscle satellite cells during chondrogenic differentiation in vitro as well as in the adult fracture healing process in vivo, suggesting that Nkx3.2 may also be involved in a cell fate determination process at a stage later than early embryogenesis. Furthermore, we show that Nkx3.2 acts as a transcriptional repressor to inhibit Pax3 promoter activity. While there are consensus Nkx3.2 binding sites on the Pax3 promoter, we have not determined whether Nkx3.2 binds to the Pax3 promoter. Interestingly, Nkx3.2 has also been shown to act as a repressor to inhibit osteogenic determining factor Runx2, suggesting that Nkx3.2 may be used to inhibit other noncartilage cell fates. We have also uncovered a pivotal role for Nkx3.2 in the induction of chondrogenic genes. We found that without the repressing activity of Nkx3.2, Sox9, despite its ability to bind to collagen II and aggrecan promoters, was unable to activate those genes or inhibit myogenesis. Additionally, Nkx3.2 potentiates the ability of Sox9 to induce aggrecan expression, which may be due to its repression of chondrogenic inhibitor Pax3. This data is consistent with the time course experiment, which indicated that the high level expression of collagen II and aggrecan is clearly correlated with the induction of Nkx3.2, as Sox9 expression is reduced at later stages of chondrogenesis.
Inactivating mutations of Nkx3.2 in human lead to spondylo-megaepiphyseal-metaphyseal dysplasia, a disease that causes abnormalities of the vertebral bodies, limbs and joints. Here we show that Nkx3.2 is activated in the muscle satellite cells during chondrogenic differentiation in vitro as well as in the adult fracture healing process in vivo, suggesting that Nkx3.2 may also be involved in a cell fate determination process at a stage later than early embryogenesis. Furthermore, we show that Nkx3.2 acts as a transcriptional repressor to inhibit Pax3 promoter activity. While there are consensus Nkx3.2 binding sites on the Pax3 promoter, we have not determined whether Nkx3.2 binds to the Pax3 promoter. Interestingly, Nkx3.2 has also been shown to act as a repressor to inhibit osteogenic determining factor Runx2, suggesting that Nkx3.2 may be used to inhibit other noncartilage cell fates. We have also uncovered a pivotal role for Nkx3.2 in the induction of chondrogenic genes. We found that without the repressing activity of Nkx3.2, Sox9, despite its ability to bind to collagen II and aggrecan promoters, was unable to activate those genes or inhibit myogenesis. Additionally, Nkx3.2 potentiates the ability of Sox9 to induce aggrecan expression, which may be due to its repression of chondrogenic inhibitor Pax3. This data is consistent with the time course experiment, which indicated that the high level expression of collagen II and aggrecan is clearly correlated with the induction of Nkx3.2, as Sox9 expression is reduced at later stages of chondrogenesis.
Concurrently with the induction of cartilage genes which should be consistent with a transdifferentiation process
Msx1 is correlated with muscle cell dedifferentiation. However, msx1 is also highly expressed in chondrocytes and is induced by BMP/TGF? signaling. Thus, although we observed a significant induction of msx1 expression upon chondrogenic differentiation in the satellite cells, it does not indicate whether the satellite cells have undergone dedifferentiation. Regardless, our data support that muscle progenitor cells that normally would undergo myogenesis, can be redirected to adopt a cartilage cell fate in vitro and in vivo. In this study, we have evaluated cartilage gene expression in the muscle progenitor cells that contribute to fracture healing. However, other cell types located in the vicinity of bone may also participate in cartilage and bone formation. Elegant grafting experiments using LacZ-positive donor mice and Lac-Z-negative recipients revealed that cells from the perichondrium, the fibrous covering of the bone, differentiate into chondrocytes and osteocytes during fracture Catharanthine sulfate repair. Cells associated with blood vessels, such as pericytes, have also been shown to have the ability to differentiate into chondrocytes. Cells that are positive for Tie-2, an endothelial cell marker, while not yet shown to be recruited to the fracture callus, are known to contribute to cartilage and bone formation during heterotopic ossification. Because of the diverse cell types that participate in cartilage formation during fracture healing, it is likely that these different types of cells use different signaling mechanisms when undergoing chondrogenic differentiation. It is known that TGF?, BMP, PTH, as well as Wnt signaling are all activated during fracture healing, and downstream molecules such as Smad, prostaglandin, Cox-2 and ?-catenin regulate this process. Our work demonstrates that transcription factors Pax3, Nkx3.2 and Sox9 regulate chondrogenic differentiation of muscle progenitor cells. However, it is unclear whether Nkx3.2 and Sox9 also participate in the chondrogenic differentiation of other cell types, such as perichondrial or endothelial cells, and how these different cell types coordinate their signaling events during fracture healing. The understanding of such signaling processes in different cell types may help to accelerate fracture healing. Pax3, Nkx3.2 and Sox9 are all known to play important roles during development. In embryogenesis, Pax3 is expressed in the dermomyotome of the somite, which gives rise to muscle cell precursors. Pax3 mutant mice exhibit somite truncations with loss of hypaxial dermomyotome, and absence of limb muscle. Our data support the role of Pax3 in promoting 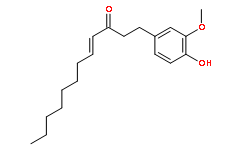 myogenesis in muscle satellite cells. Furthermore, our data shows that Pax3 has an additional function of inhibiting chondrogenic differentiation of muscle satellite cells. It was reported that constitutive expression of Pax3 led to increased proliferation and decreased cell size in satellite cells. We found that Pax3-infected cells had a much more elongated appearance as compared to the control cells cultured in the chondrogenic medium, although we could not clearly distinguish the differences in cell shape due to cell condensation that accompanies chondrogenesis. In the double knockout of Pax3 and its paralogue Pax7, significant cell death takes place, leading to the loss of most muscle fibers. In addition, Pax3 and Pax7 double mutant cells were found in the forming rib, Pimozide suggesting that they may have adopted a cartilage fate.
myogenesis in muscle satellite cells. Furthermore, our data shows that Pax3 has an additional function of inhibiting chondrogenic differentiation of muscle satellite cells. It was reported that constitutive expression of Pax3 led to increased proliferation and decreased cell size in satellite cells. We found that Pax3-infected cells had a much more elongated appearance as compared to the control cells cultured in the chondrogenic medium, although we could not clearly distinguish the differences in cell shape due to cell condensation that accompanies chondrogenesis. In the double knockout of Pax3 and its paralogue Pax7, significant cell death takes place, leading to the loss of most muscle fibers. In addition, Pax3 and Pax7 double mutant cells were found in the forming rib, Pimozide suggesting that they may have adopted a cartilage fate.
The enhancement of keratinocyte proliferation was also significantly reduced since the production of IL-1a
TGFb1 by fibroblasts was inhibited. HB-EGF was also reduced in cultures with IL-1a or TGFb1 siRNA-transfected fibroblasts and in co-cultures with antiIL-1a or anti-TGFb1 antibodies, indicating that the keratinocyte proliferation upregulated by IL-1a and TGFb1 in the co-culture was partially mediated by HB-EGF. The effects of the anti-HBEGF antibody on keratinocyte proliferation were significantly greater than those of the anti-IL-1a or anti-TGFb1 antibodies, suggesting that HB-EGF plays a central role in the regulation of kerotinocyte growth in these co-cultures. Since siRNA can only partially or/and Tulathromycin B transiently block target cytokine production and the inhibition antibodies we used in our experiments were all overdosage, the inhibitory effects of siRNA was always weaker than those of inhibition antibodies in our observations. After the early stage of the co-culture, the keratinocytes in all of the culture conditions proliferated at similar rates, with the exception of the EGF control. The EGF culture showed faster late stage growth than the other cultures, suggesting a reduction of cell growth factors in these cultures. However, when the concentrations of the HB-EGF, IL1a, and TGFb1 were examined, it was found that they continued to increase as the cells grew. Further analysis into the ratio of HBEGF levels and cell count revealed that the increase in HB-EGF, IL-1a, and TGFb1 levels failed to match the growth rate, resulting in lower levels of these cytokines per cell during the later stage 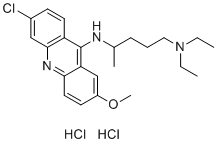 of the culture. Both keratinocytes and fibroblasts have very high motility in culture, which enables them to meet and form cell-to-cell contacts even when co-cultured at very low densities. To confirm the effects of cell contact on keratinocyte proliferation and migration that was promoted by fibroblasts and relevant cytokines, transwell plates were used to separate the two cell types in this study. The two cell types were separated into the upper and lower chambers of the transwell to eliminate Gomisin-D direct contact with each other, and the cell type being observed was placed in the lower chamber. The data clearly showed that without direct contact, the fibroblasts and the IL-1a and TGFb1 cytokines produced by the fibroblasts did not have a significant effect on the proliferation and migration of co-cultured keratinocytes. To further confirm the effects of IL-1a, TGFb1 and HB-EGF on keratinocyte proliferation, these cytokines were added to keratinocyte cultures at different concentrations and compared to the kerotinocye/ fibroblast co-culture. The results showed that all three cytokines were able to stimulate keratinocyte proliferation, but require a 10fold higher concentration of the cytokines, which suggested that since the overall cytokine level of the culture was insufficient to account for the observed effects, direct contact may be required to provide a microenvironment with sufficient cytokine levels. Cell death could be a factor that affects the evaluation of cell proliferation by cell counting. To eliminate the interference of cell death on our experimental results, we did Anexin V binding assay for some samples in addition to our routine Trypan Blue Exclusion test for all samples. Trypan Blue Exclusion was assessed on days 5, 10 and 15, when the cells were harvested. Organ tissues are comprised of groups of cells that coordinate their activities so as to achieve a functional outcome. In bone, the functional unit of cells is called a ‘basic multicellular unit’. BMUs are transient functional groupings of cells that progress through the bone.
of the culture. Both keratinocytes and fibroblasts have very high motility in culture, which enables them to meet and form cell-to-cell contacts even when co-cultured at very low densities. To confirm the effects of cell contact on keratinocyte proliferation and migration that was promoted by fibroblasts and relevant cytokines, transwell plates were used to separate the two cell types in this study. The two cell types were separated into the upper and lower chambers of the transwell to eliminate Gomisin-D direct contact with each other, and the cell type being observed was placed in the lower chamber. The data clearly showed that without direct contact, the fibroblasts and the IL-1a and TGFb1 cytokines produced by the fibroblasts did not have a significant effect on the proliferation and migration of co-cultured keratinocytes. To further confirm the effects of IL-1a, TGFb1 and HB-EGF on keratinocyte proliferation, these cytokines were added to keratinocyte cultures at different concentrations and compared to the kerotinocye/ fibroblast co-culture. The results showed that all three cytokines were able to stimulate keratinocyte proliferation, but require a 10fold higher concentration of the cytokines, which suggested that since the overall cytokine level of the culture was insufficient to account for the observed effects, direct contact may be required to provide a microenvironment with sufficient cytokine levels. Cell death could be a factor that affects the evaluation of cell proliferation by cell counting. To eliminate the interference of cell death on our experimental results, we did Anexin V binding assay for some samples in addition to our routine Trypan Blue Exclusion test for all samples. Trypan Blue Exclusion was assessed on days 5, 10 and 15, when the cells were harvested. Organ tissues are comprised of groups of cells that coordinate their activities so as to achieve a functional outcome. In bone, the functional unit of cells is called a ‘basic multicellular unit’. BMUs are transient functional groupings of cells that progress through the bone.