These results indicate for the first time, that the observed lower mitochondrial content in muscle of septic ICU patients with multiple organ failure is not due to a general decrease in mitochondrial gene expression or failure in the protein synthesis process. The balance between the biogenesis pathways and the rate of mitochondrial protein degradation regulates tissue mitochondrial content. The coordinate gene expression for the estimated 1500 or so mitochondrial proteins, is under tight control by these Albaspidin-AA different transcriptional regulators. The nuclear encoded mitochondrial proteins are regulated by NRF1, NRF2a/GABP as well as PGC1a and b, while mitochondrial DNA replication and transcription is greatly influenced by TFAM, TFB1M and TFB2M. In the present study we found high mRNA levels of all three transcriptional regulators that act on mitochondrial DNA, and intriguingly selectively high NRF2a/ GABP levels, a transcription factor that regulates the nuclear encoded TFAM; TFB1M and TFB2M. We also determined that the selective activation of NRF2a/GABP was unlikely to be caused by exogenous insulin. The inability of mitochondrial protein synthesis to 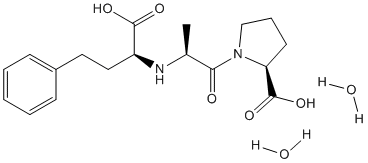 sustain mitochondrial capacity may therefore reflect a lack of coordinated expression of all the transcription factors needed for an increased mitochondrial biogenesis. The reason for the lack of coordinated increase in mitochondrial biogenesis genes in these patients needs to be elucidated and may ultimately be manipulated to improve patient outcome. It has previously been reported that mitochondrial protein synthesis and gene expression are decreased in septic rats. Like our present comparative analysis, this supports the idea that Mechlorethamine hydrochloride rodent models may be inappropriate for studying mitochondrial dynamics. The unchanged mitochondrial protein synthesis rates are in line with the synthesis rates of other muscle proteins in the septic ICU patients. The very characteristic loss of total muscle protein in these patients is also accompanied by normal synthesis rates of total muscle protein while our transcriptomics indicates that the actual proteins being made, will clearly be substantially different from control skeletal muscle. Future detailed proteomic analyses combined with ribosomal RNA analysis will allow us to define which proteins are now being synthesised. The translational initiation factors are central components of the protein translation machinery and EIF4 is the proposed rate limiting factor for protein translational. While the partners of EIF4E, EIF4A1, EIF4G1 and EIF3S10 were all up-regulated in the ICU patients, EIF4E is unchanged at the mRNA level. In combination with the increased expression of the tRNA synthetases, MARS, LARS and RARS it would appear that a substantial yet incomplete attempt was made to promote muscle protein synthesis, similar to that observed for the mitochondria. This appears to have failed perhaps reflecting a lack of up-regulation of EIF4E, however, EIF4E is not considered to be regulated to a great extent, at the mRNA level, and thus further analysis is merited. Overall, it is clear that the lack of gross changes in in vivo protein dynamics must obscure critical alterations in specific protein formation, such that combing protein measurements with global transcriptomics provides a powerful solution to understanding the nature of tissue remodelling in multiple organ failure patients. When looking for novel regulators of altered protein production from mRNA, microRNA��s as global regulators of protein synthesis are of great interest.
sustain mitochondrial capacity may therefore reflect a lack of coordinated expression of all the transcription factors needed for an increased mitochondrial biogenesis. The reason for the lack of coordinated increase in mitochondrial biogenesis genes in these patients needs to be elucidated and may ultimately be manipulated to improve patient outcome. It has previously been reported that mitochondrial protein synthesis and gene expression are decreased in septic rats. Like our present comparative analysis, this supports the idea that Mechlorethamine hydrochloride rodent models may be inappropriate for studying mitochondrial dynamics. The unchanged mitochondrial protein synthesis rates are in line with the synthesis rates of other muscle proteins in the septic ICU patients. The very characteristic loss of total muscle protein in these patients is also accompanied by normal synthesis rates of total muscle protein while our transcriptomics indicates that the actual proteins being made, will clearly be substantially different from control skeletal muscle. Future detailed proteomic analyses combined with ribosomal RNA analysis will allow us to define which proteins are now being synthesised. The translational initiation factors are central components of the protein translation machinery and EIF4 is the proposed rate limiting factor for protein translational. While the partners of EIF4E, EIF4A1, EIF4G1 and EIF3S10 were all up-regulated in the ICU patients, EIF4E is unchanged at the mRNA level. In combination with the increased expression of the tRNA synthetases, MARS, LARS and RARS it would appear that a substantial yet incomplete attempt was made to promote muscle protein synthesis, similar to that observed for the mitochondria. This appears to have failed perhaps reflecting a lack of up-regulation of EIF4E, however, EIF4E is not considered to be regulated to a great extent, at the mRNA level, and thus further analysis is merited. Overall, it is clear that the lack of gross changes in in vivo protein dynamics must obscure critical alterations in specific protein formation, such that combing protein measurements with global transcriptomics provides a powerful solution to understanding the nature of tissue remodelling in multiple organ failure patients. When looking for novel regulators of altered protein production from mRNA, microRNA��s as global regulators of protein synthesis are of great interest.