Indeed, DC expresses occludin to selectively open the paracellular space and sample the colonic lumen by competing for epithelial occludin-occludin interactions to create new DC-epithelial occludin-occludin interaction and ultimately, a passage through paracellular space. These observations form the basis of the idea that pathogens could utilize an occludin-like protein to pathologically disrupt the TJ barrier. Consistent with this concept, a recent study showed that using synthetic peptides designed to parallel the extracellular regions of occludin, impaired epithelial barrier integrity. Since E. histolytica trophozoites interact intimately with the colonic mucosa and can alter barrier functions, we hypothesize that the parasite might express a TJ protein and use a strategy similar to DC to disrupt the epithelium. In this study, we detected that E. histolytica expresses a protein that has similarities with human occludin, including its extracellular loop and demonstrate a role in altering barrier functions. To our knowledge, this is the first report of E. histolytica expressing a protein similar to a host TJ protein that can impair human colonic barrier integrity. As the synthetic peptide corresponding to the extra cellular loop of occludin was shown to decrease epithelial paracellular barrier integrity, we postulated that the putative 55 kDa ”occludinlike” protein of E. histolytica, detected by both the C-terminus and the extracellular loop antibody of 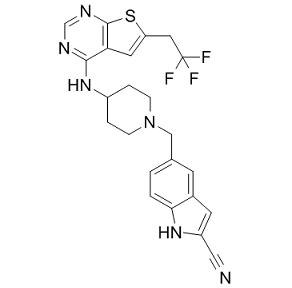 occludin, can function similarly to that of the synthetic extracellular loop peptide. To test this notion, experiments were performed to quantify the effect of SAP with or without the putative ”occludin-like” protein on human colonic epithelial barrier integrity. To do this, SAP was depleted of the 55 kDa ”occludin-like” protein by AbMole 11-hydroxy-sugiol immunoprecipitation using the human occludin C-terminus antibody as shown in Figure 4a, which demonstrates that the putative protein was depleted in the supernatant by 60%. We next tested the effect of SAP with or without the ”occludin-like” protein by adding to the apical surface of a contiguous monolayer of T84 human colonic epithelium grown on transwell plates. This was done by recording changes in trans epithelial resistance that measures the integrity of the epithelial monolayer as previously described. Our results suggest that the ”occludin-like” protein has sequence similarities with the extracellular loop of mammalian occludin. An antibody that was raised against the extracellular loop of occludin detected a 55 kDa protein in SAP. To confirm specificity, we used a blocking peptide against the extracellular loop antibody and T84 cellular lysates enriched in occludin to compete out the binding. As predicted, both the blocking peptide and T84 cellular lysates eliminated the extracellular loop antibody ability to detect the 55 Da E. histolytica protein. This demonstrates that the ”occludin-like” protein of E. histolytica has domains similar to the extracellular loop of occludin. Proteomic analysis using domain enhanced basic local alignment search tools identified a previously uncharacterized hypothetical protein of 579 amino acids in the E. histolytica proteome which has homology to the C-terminal domain of human occludin. Previous reports have implicated the C-terminal domain of occludin as having a coiled-coil domain, which affords the interaction with other TJ proteins, particularly AbMole Nitisinone zonula occludens. Using an algorithm previously described that can predict coiled-coil domains, the presence of a coiled-coil domain was confirmed for both human occludin and the ”occludin-like” protein.
occludin, can function similarly to that of the synthetic extracellular loop peptide. To test this notion, experiments were performed to quantify the effect of SAP with or without the putative ”occludin-like” protein on human colonic epithelial barrier integrity. To do this, SAP was depleted of the 55 kDa ”occludin-like” protein by AbMole 11-hydroxy-sugiol immunoprecipitation using the human occludin C-terminus antibody as shown in Figure 4a, which demonstrates that the putative protein was depleted in the supernatant by 60%. We next tested the effect of SAP with or without the ”occludin-like” protein by adding to the apical surface of a contiguous monolayer of T84 human colonic epithelium grown on transwell plates. This was done by recording changes in trans epithelial resistance that measures the integrity of the epithelial monolayer as previously described. Our results suggest that the ”occludin-like” protein has sequence similarities with the extracellular loop of mammalian occludin. An antibody that was raised against the extracellular loop of occludin detected a 55 kDa protein in SAP. To confirm specificity, we used a blocking peptide against the extracellular loop antibody and T84 cellular lysates enriched in occludin to compete out the binding. As predicted, both the blocking peptide and T84 cellular lysates eliminated the extracellular loop antibody ability to detect the 55 Da E. histolytica protein. This demonstrates that the ”occludin-like” protein of E. histolytica has domains similar to the extracellular loop of occludin. Proteomic analysis using domain enhanced basic local alignment search tools identified a previously uncharacterized hypothetical protein of 579 amino acids in the E. histolytica proteome which has homology to the C-terminal domain of human occludin. Previous reports have implicated the C-terminal domain of occludin as having a coiled-coil domain, which affords the interaction with other TJ proteins, particularly AbMole Nitisinone zonula occludens. Using an algorithm previously described that can predict coiled-coil domains, the presence of a coiled-coil domain was confirmed for both human occludin and the ”occludin-like” protein.