Certain IDC have been proven to be potent inhibitors of HIV-1 replication in cell culture through a selective action on exonic splicing enhancers -dependent activity of individual SR proteins. One such molecule, IDC16 has been shown to interfere with the SF2/ASF SR protein and production of HIV regulatory proteins and to compromise assembly of infectious particles. However, no evaluation of IDC on retrovirus-mediated pathogenesis has yet been documented. Here, we have taken advantage of the F-MLV induced pathogenesis model in newborn mice to evaluate the efficiency of this new class of molecules at different stages of retrovirus infection and disease. We show now that different IDC differentially inhibit HIV-1 and MLV, most likely reflecting distinct requirement for cellular splicing factors. Thus, we found that IDC13 and IDC78, but not IDC16, prevented F-MLV replication both ex vivo and in vivo, by selectively altering single-splicing of the retroviral genome. Furthermore, we describe two IDC that also proved to be very efficient at protecting mice from MLV-induced pathogenesis by inhibiting early viral replication. In order to better understand the molecular mechanisms underlying the specific inhibition of MLV replication by some of the IDC, we analyzed the viral RNA content of infected cells. Dunni cells were infected with F-MLV in the presence of different IDC and total RNA was extracted and used as template for RTPCR. We used two different sets of oligonucleotide primers that allowed us to discriminate between spliced and unspliced viral RNAs. As an internal control, RT-PCR was performed on mRNA from the gadph house-keeping gene. Compared to untreated cells, accumulation of the PCR product corresponding to the spliced F-MLV RNA dramatically decreased upon treatment with IDC13 and IDC78, while accumulation of the gapdh product did not decrease. Neither IDC16, mentioned above, nor IDC217, a compound that had no MK-0683 molecular weight effect on all splicing substrates tested, had detectable impact 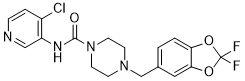 on F-MLV splicing. Altogether, these results indicated that inhibition of F-MLV replication by certain IDC appeared directly associated with their ability to specifically inhibit viral RNA splicing, an event required for expression of the viral Env glycoprotein. However, we observed that the significant decrease of spliced product observed after IDC13 and IDC78 treatment was not compensated by a corresponding increase of unspliced FMLV RNA. Instead, we noted that IDC13, IDC78 and also IDC16 affected, albeit to a lesser extent for the latter, accumulation of unspliced viral RNA. These results suggested that IDC13 and IDC78 inhibited F-MLV replication by altering viral RNA splicing, but that other pathway governing RNA accumulation, such as transcriptional levels, RNA trafficking and/or RNA stability, could also be altered. Furthermore, we observed that there was a correlation between a lower plasmatic viremia and increased latency of disease in CPI-613 IDC-treated mice. Altogether, this indicated that lower virulence of F-MLV, observed in IDC-treated mice, was likely due to inhibition of virus replication during the early phase of the disease. In order to further assess whether resistance to F-MLV-induced erythroleukemia in IDC-treated mice was indeed due to inhibition of earlyvirus dissemination, and nottoa toxic effect leadingtoreduction of target cells, we measured the direct effect of these compounds on erythroid differentiation in vivo. Newborn mice were injected with compounds IDC13, IDC78 or IDC217and followed both for hematocrits between 16 to 21 days of age and for spleen enlargement as an indication of compensatory splenic activity.
on F-MLV splicing. Altogether, these results indicated that inhibition of F-MLV replication by certain IDC appeared directly associated with their ability to specifically inhibit viral RNA splicing, an event required for expression of the viral Env glycoprotein. However, we observed that the significant decrease of spliced product observed after IDC13 and IDC78 treatment was not compensated by a corresponding increase of unspliced FMLV RNA. Instead, we noted that IDC13, IDC78 and also IDC16 affected, albeit to a lesser extent for the latter, accumulation of unspliced viral RNA. These results suggested that IDC13 and IDC78 inhibited F-MLV replication by altering viral RNA splicing, but that other pathway governing RNA accumulation, such as transcriptional levels, RNA trafficking and/or RNA stability, could also be altered. Furthermore, we observed that there was a correlation between a lower plasmatic viremia and increased latency of disease in CPI-613 IDC-treated mice. Altogether, this indicated that lower virulence of F-MLV, observed in IDC-treated mice, was likely due to inhibition of virus replication during the early phase of the disease. In order to further assess whether resistance to F-MLV-induced erythroleukemia in IDC-treated mice was indeed due to inhibition of earlyvirus dissemination, and nottoa toxic effect leadingtoreduction of target cells, we measured the direct effect of these compounds on erythroid differentiation in vivo. Newborn mice were injected with compounds IDC13, IDC78 or IDC217and followed both for hematocrits between 16 to 21 days of age and for spleen enlargement as an indication of compensatory splenic activity.