Inhibition of Src leads to cell cycle arrest and reduction of migration and inhibition. Dual inhibition of Src and Etk are expected to inhibit growth, migration, and survival of the cells. To the best of our knowledge, this is the first selective Etk/Src inhibitor reported to date. CTA095 belongs to a novel class of kinase inhibitors with a chemical structure distinct from other known TKIs. This inhibitor is most potent in inhibiting Etk followed by Src, but it is not a potent inhibitor for other Tec and Src family kinases such as Btk, Yes and Lyn. Treatment of PC3 cells with CTA095 resulted in the decrease of phoshorylation of Etk and Src, as well as the downstream signals Stat3 and Akt. CTA095 has little effect on the survival of the immortalized normal prostate cell RWPE1, suggesting that the growth and survival of prostate cancer cells are more dependent on Etk and Src signals, consistent with the observed overexpression of Etk in prostate cancer tissues and the development of PIN phenotype in transgenic animals carrying overexpressed Etk. Etk interacts with and inactivates p53, and overexpression of Etk in prostate cancer cells confers resistance to androgen deprivationand photodynamic therapy. On the other hand, truncated Etk, due to caspase cleavage, induces apoptosis. This data taken together suggests that Etk is a strong regulator of apoptosis. At the same time, CTA095 was found to induce autophagy in human prostate cancer cells. Autophagy is generally regarded as a pro-survival mechanism, where wasted proteins and retired organs are degraded to regenerate energy during stress conditions. It is inhibited by energy sensor 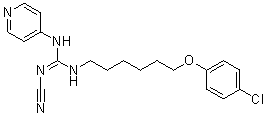 mTOR, a target of rapamycin. Excessive autophagy, under special conditions, however, can lead to programmed cell death. Previously, we reported that autophagy induced by Src inhibitor is the underlying cause of apoptosis-resistance of the GW786034 treated cells. These cells are growth arrested, but do not undergo apoptosis. Blocking autophagy by siRNA BKM120 targeting of autophagy components, or the simultaneous application of CQ, an inhibitor of autophagy flow, results in massive cell death. It is interesting that, like the Src inhibitor, CTA095 induced autophagy is likely due to its inhibition of mTOR activation. However, unlike the Src inhibitor, CTA095 inhibits both Etk and Src, and can overcome the apoptosis resistance induced by autophagy. This data suggests that the absence of Etk proactively turns on the apoptosis pathway, which cannot be completely overridden by the restoring act of autophagy. The finding that the autophagy inhibitor CQ further enhances the apoptosis-inducing effect of CTA095 suggests that autophagy contributes partially to the survival of CTA095 treated cells. Alternatively, CQ is known to have other cellular effects including induction of p53 and intercalation of DNA, which may contribute additional toxicity to the treated cells. Curiously, we found that the autophagy inducer RPM also synergizes with CTA095, suggesting excessive autophagy may contribute additional means to kill prostate cancer cells. CTA095 was also a chemo-sensitizer and showed synergistic effect with PTX, indicating its role in combination therapy for prostate cancer. In addition to these findings, CTA095 was found to inhibit HUVEC cell tube formation and “wound healing” of prostate cancer cells, implying its role in inhibition of angiogenesis and metastasis of human prostate cancer cells. More interestingly, simultaneously targeting of Etk and Src by CTA095 overcomes the Src inhibitor resistance in prostate cancer cells. The Src inhibitor AZD0530 has a good effect on inhibition of bone metastasis of prostate cancer, but lacks the ability to induce apoptosis in prostate cancer cells.
mTOR, a target of rapamycin. Excessive autophagy, under special conditions, however, can lead to programmed cell death. Previously, we reported that autophagy induced by Src inhibitor is the underlying cause of apoptosis-resistance of the GW786034 treated cells. These cells are growth arrested, but do not undergo apoptosis. Blocking autophagy by siRNA BKM120 targeting of autophagy components, or the simultaneous application of CQ, an inhibitor of autophagy flow, results in massive cell death. It is interesting that, like the Src inhibitor, CTA095 induced autophagy is likely due to its inhibition of mTOR activation. However, unlike the Src inhibitor, CTA095 inhibits both Etk and Src, and can overcome the apoptosis resistance induced by autophagy. This data suggests that the absence of Etk proactively turns on the apoptosis pathway, which cannot be completely overridden by the restoring act of autophagy. The finding that the autophagy inhibitor CQ further enhances the apoptosis-inducing effect of CTA095 suggests that autophagy contributes partially to the survival of CTA095 treated cells. Alternatively, CQ is known to have other cellular effects including induction of p53 and intercalation of DNA, which may contribute additional toxicity to the treated cells. Curiously, we found that the autophagy inducer RPM also synergizes with CTA095, suggesting excessive autophagy may contribute additional means to kill prostate cancer cells. CTA095 was also a chemo-sensitizer and showed synergistic effect with PTX, indicating its role in combination therapy for prostate cancer. In addition to these findings, CTA095 was found to inhibit HUVEC cell tube formation and “wound healing” of prostate cancer cells, implying its role in inhibition of angiogenesis and metastasis of human prostate cancer cells. More interestingly, simultaneously targeting of Etk and Src by CTA095 overcomes the Src inhibitor resistance in prostate cancer cells. The Src inhibitor AZD0530 has a good effect on inhibition of bone metastasis of prostate cancer, but lacks the ability to induce apoptosis in prostate cancer cells.