This is not surprising considering the two forms are distinct in all respects except their ability to oxidize glycerol 3-phosphate to DHAP. However, this enzyme specificity suggests that iGPs are likely not acting as inhibitory analogs in the substrate binding pocket as described for non-selective inhibitors of both forms such as glyceraldehyde 3-phosphate. The MDV3100 observation of increased Km as well as the lower values for Kic than Kiu indicates that both iGP-1 and iGP-5 have a greater affinity for free enzyme. Both inhibitors have Hill slopes near unity, suggesting they interact with mGPDH at a single, allosteric binding site. Although the analysis of inhibition kinetics was performed in the presence of activating calcium, our evidence from assays of mGPDH-specific H2O2 production and DYm driven by glycerol phosphate suggest that iGPs act independently of the calcium-sensing mechanism of mGPDH. We cannot rule out direct interactions between iGPs and either the FAD binding domain in the soluble portion of mGPDH or the ubiquinone binding 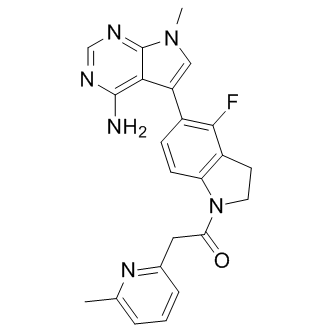 pocket embedded in the outer leaflet of the inner mitochondrial membrane. The interaction with the ubiquinone pocket might be tested by studies similar to those presented here but with differing ubiquinol as the electron donor to the enzyme. Future co-crystallization structural studies and enzymatic assays of iGPs with the bacterial or mammalian FAD-linked GPDH may provide the best opportunities to identify the exact mode of interaction and mechanism of action of these novel inhibitors. As initial confirmed hits in a small-molecule screen, our most promising iGPs demonstrate excellent potency and good selectivity. Apart from a subtle effect on succinate oxidation at high concentrations, iGP-1 does not alter mitochondrial oxidation of numerous substrates including a second dicarboxylate, malate. Therefore, it is unlikely that the subtle effect on succinate oxidation is due to inhibition of the dicarboxylate transporter by the succinamide of iGPs. Indeed, analogs iGP-17�C iGP-19 retain the succinamide without or with the attached phenyl ring yet do not alter succinate oxidation as assessed by H2O2 production by succinate alone. This suggests at least partial dependence on the benzimidazole ring system for the subtle effect of iGP-1 on succinate oxidation, perhaps via direct interaction with complex II. Reactions of the tricarboxylic acid cycle shared between succinate, malate, and pyruvate oxidation also do not appear to be affected by iGP-1. Further, iGP-1 shows no effects on the maintenance of proton motive force or rates of ATP synthesis with substrates other than glycerol phosphate. In addition, we can infer from the synaptosomal experiments that iGP-1 does not prevent pyruvate uptake into cells or mitochondria and does not directly alter glycolysis. Therefore, our data identify an exemplary inhibitor that is both potent and selective against mGPDH and offers structural targets through which additional improvements to these activities can be BKM120 structure achieved. In conclusion, we have identified a novel class of potent, selective, cell-permeant inhibitors of mGPDH that act via mixed inhibition. Further tests of the role of mGPDH and glycerol phosphate shuttle activities under conditions of neuronal activity or in other cell types with differing shuttle capacities will help determine those in which mGPDH activity is essential. Our novel inhibitors of mGPDH provide means to test these possibilities pharmacologically. The ubiquitin�Cproteasome system is the major protein degradation pathway in every cell. The eukaryotic proteasome is a potential target for antitumor drugs. To date, two proteasome inhibitors, the Bortezomib and Carfilzomib, have been approved for the treatment of multiple myeloma.
pocket embedded in the outer leaflet of the inner mitochondrial membrane. The interaction with the ubiquinone pocket might be tested by studies similar to those presented here but with differing ubiquinol as the electron donor to the enzyme. Future co-crystallization structural studies and enzymatic assays of iGPs with the bacterial or mammalian FAD-linked GPDH may provide the best opportunities to identify the exact mode of interaction and mechanism of action of these novel inhibitors. As initial confirmed hits in a small-molecule screen, our most promising iGPs demonstrate excellent potency and good selectivity. Apart from a subtle effect on succinate oxidation at high concentrations, iGP-1 does not alter mitochondrial oxidation of numerous substrates including a second dicarboxylate, malate. Therefore, it is unlikely that the subtle effect on succinate oxidation is due to inhibition of the dicarboxylate transporter by the succinamide of iGPs. Indeed, analogs iGP-17�C iGP-19 retain the succinamide without or with the attached phenyl ring yet do not alter succinate oxidation as assessed by H2O2 production by succinate alone. This suggests at least partial dependence on the benzimidazole ring system for the subtle effect of iGP-1 on succinate oxidation, perhaps via direct interaction with complex II. Reactions of the tricarboxylic acid cycle shared between succinate, malate, and pyruvate oxidation also do not appear to be affected by iGP-1. Further, iGP-1 shows no effects on the maintenance of proton motive force or rates of ATP synthesis with substrates other than glycerol phosphate. In addition, we can infer from the synaptosomal experiments that iGP-1 does not prevent pyruvate uptake into cells or mitochondria and does not directly alter glycolysis. Therefore, our data identify an exemplary inhibitor that is both potent and selective against mGPDH and offers structural targets through which additional improvements to these activities can be BKM120 structure achieved. In conclusion, we have identified a novel class of potent, selective, cell-permeant inhibitors of mGPDH that act via mixed inhibition. Further tests of the role of mGPDH and glycerol phosphate shuttle activities under conditions of neuronal activity or in other cell types with differing shuttle capacities will help determine those in which mGPDH activity is essential. Our novel inhibitors of mGPDH provide means to test these possibilities pharmacologically. The ubiquitin�Cproteasome system is the major protein degradation pathway in every cell. The eukaryotic proteasome is a potential target for antitumor drugs. To date, two proteasome inhibitors, the Bortezomib and Carfilzomib, have been approved for the treatment of multiple myeloma.