It was not detected in the globular embryo stage. Interestingly, few of previous embryo-related proteomic studies have reported storage protein of napin, in contrast to that cruciferin is frequently detected. Expression of a novel protein, AKT2/3, increased in the early stage of seed development and began to decrease after 20 DAP. In Arabidopsis, AKT2/3 encodes photosynthateand light-dependent inward rectifying potassium channel with unique gating properties that are 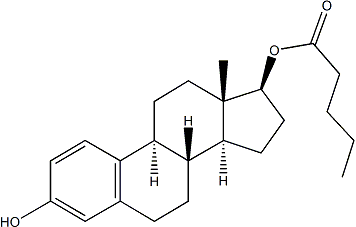 regulated by phosphorylation. Therefore, identification of AKT2/3 Tulathromycin B suggested its novel role in seed development. Another interesting finding comes from an unclassified protein TSJT1, which has been indicated as stem-specific and found in gene chip data. During seed development, it was very highly expressed early at 10 DAP, but after 20 DAP, its protein significantly decreased, therefore, our analysis indicates it may be important for early seed development, which remains to be determined by further experiment. An investigation on seed development should significantly enrich our knowledge on the molecular and physiological events in whole seed growth process. In this study, we explored the protein dynamics over five stages during B. campestri seed development using a proteomic approach. A total of 209 proteins were identified by mass spectrometry to be differentially seed development and they could be classified into 16 functional groups. It was found that proteins participating in metabolism, energy production, oxidation/detoxification as well as stress/ defense were highly dynamic in abundance. However, expressed during functional assignment of these altered proteins uncovers unexpected abundance of proteins related to protein processing and destination, highlighting the importance of protein renewal in seed development, and proportion of those associated to cell structure was rather low compared to previous proteomic analysis of seed development. Our study provides important information to better understanding the seed development in oil plant. The catalytic a2 subunits were produced in reduced amounts as soluble His-tagged proteins and in larger quantities as non-tagged proteins in inclusion bodies, from which they were refolded and purified. Despite the high degree of identity among a1 and a2 amino acid sequences the protocol developed for refolding of a1 subunits had to be slightly modified. These modifications refer to an increase in the solubilization time and the first refolding step. Better yields were obtained when the dilution at this step was made to 1 M denaturant, and hence to a lower protein concentration. AdoMet synthesis requires production of oligomers with the correct orientation of their subunits, since catalytic sites locate at the interface of the monomers in the dimer with residues of each contributing to them. Therefore, Orbifloxacin analytical gel filtration chromatography of refolded-a2 was carried out and the protein detected by activity measurements and Dot-Blot. The active protein exhibited an elution volume of 12.5 ml that corresponded to an 87 kDa oligomer, according to the elution profile of the markers. The calculated size is compatible with a dimeric association state, a result that is in agreement with previous data obtained for recombinant a2. However, injection of refolded-a2 samples at higher protein concentrations revealed the presence of two peaks, by both activity and Dot-blot, with calculated elution volumes of 11.44 and 12.50 ml, compatible with tetrameric and dimeric forms.
regulated by phosphorylation. Therefore, identification of AKT2/3 Tulathromycin B suggested its novel role in seed development. Another interesting finding comes from an unclassified protein TSJT1, which has been indicated as stem-specific and found in gene chip data. During seed development, it was very highly expressed early at 10 DAP, but after 20 DAP, its protein significantly decreased, therefore, our analysis indicates it may be important for early seed development, which remains to be determined by further experiment. An investigation on seed development should significantly enrich our knowledge on the molecular and physiological events in whole seed growth process. In this study, we explored the protein dynamics over five stages during B. campestri seed development using a proteomic approach. A total of 209 proteins were identified by mass spectrometry to be differentially seed development and they could be classified into 16 functional groups. It was found that proteins participating in metabolism, energy production, oxidation/detoxification as well as stress/ defense were highly dynamic in abundance. However, expressed during functional assignment of these altered proteins uncovers unexpected abundance of proteins related to protein processing and destination, highlighting the importance of protein renewal in seed development, and proportion of those associated to cell structure was rather low compared to previous proteomic analysis of seed development. Our study provides important information to better understanding the seed development in oil plant. The catalytic a2 subunits were produced in reduced amounts as soluble His-tagged proteins and in larger quantities as non-tagged proteins in inclusion bodies, from which they were refolded and purified. Despite the high degree of identity among a1 and a2 amino acid sequences the protocol developed for refolding of a1 subunits had to be slightly modified. These modifications refer to an increase in the solubilization time and the first refolding step. Better yields were obtained when the dilution at this step was made to 1 M denaturant, and hence to a lower protein concentration. AdoMet synthesis requires production of oligomers with the correct orientation of their subunits, since catalytic sites locate at the interface of the monomers in the dimer with residues of each contributing to them. Therefore, Orbifloxacin analytical gel filtration chromatography of refolded-a2 was carried out and the protein detected by activity measurements and Dot-Blot. The active protein exhibited an elution volume of 12.5 ml that corresponded to an 87 kDa oligomer, according to the elution profile of the markers. The calculated size is compatible with a dimeric association state, a result that is in agreement with previous data obtained for recombinant a2. However, injection of refolded-a2 samples at higher protein concentrations revealed the presence of two peaks, by both activity and Dot-blot, with calculated elution volumes of 11.44 and 12.50 ml, compatible with tetrameric and dimeric forms.