In our analysis, four isoforms of E1 and eight proteasome components were observed. Folding of nascent polypeptides into functional proteins is controlled by a number of molecular chaperones and protein-folding catalysts. Our analysis revealed 6 different isoforms of protein disulphide isomerases, an endoplasmic reticulum-located protein that catalyzes the formation, isomerization, and reduction/oxidation of disulfide bonds. Seven chaperonins or chaperones were also observed, including the plant homolog of the immunoglobulin heavy-chain binding protein, which is an endoplasmic 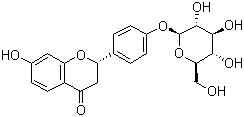 reticulum- localized member of the heat shock 70 family. BiP has been proposed to play a role in protein body assembly within the endoplasmic reticulum. These proteins displayed different accumulation patterns in the process of seed development. For example, spot 73 was identified as a protein disulfide isomerase that LOUREIRIN-B continued to accumulate and reached the highest at 20 DAP. Consistent with this in the transcript level, our gene expression analysis also revealed disulfide isomerase can be detected at the late stage of embryogenesis. Plant cysteine proteases are important for organ senescence, plant defense and nutrient mobilization during seed germination, and previous studies reveal cysteine proteinases are up-regulated in various senescing plants, such as Arabidopsis, B. napus, and Nicotiana tabacum. In this study, we identified spot 542 as senescenceassociated cysteine protease, and spot 576 as another cysteine proteinase that increased its abundance all over the five stages, suggesting that cysteine proteinase also played an important role in maturation and senescence of seed growth. Altered accumulation of these proteins indicated active protein Lomitapide Mesylate production and elimination occurred in the process of seed development, which might serve as a monitoring mechanism over those intricate processes of metabolism and energy production. It��s also highly likely that the accumulation of these proteins may be used during rapid cell division and cell structure construction. Despite of these, preponderance of these proteins seemed to be particular of our study, because few of previous reports has indicated so many proteins with similar function, which make us underestimate the importance of protein selfrenewal. Therefore, protein renewal could be an essential regulatory mechanism for seed development. The developing oilseeds take up sugars and amino acids from the surrounding endosome liquid and synthesize large quantities of triacylglycerol storage proteins. Previous work characterizes carbon assimilation during seed filling in Brassica napus and castor, both of which are oil plants. It��s interesting to examine this important metabolism pathway in the seed development. It has been demonstrated that glycolysis supplies most carbon to fatty acid synthesis in rapeseed developing embryos in culture, suggesting glycolysis is essential for carbon assimilation in the developing seeds, but relatively little is known about its regulation and control, and due to the parallel pathways operated in both the cytosol and plastids, it become more complex in plants. Our analysis identified only two storage proteins: napin and cruciferin, which are the two major storage proteins in rape seed, and constitute 20% and 60% of the total protein in mature seeds. As has been reported that their biological synthesis begins early from the expansion phase of embryo development.
reticulum- localized member of the heat shock 70 family. BiP has been proposed to play a role in protein body assembly within the endoplasmic reticulum. These proteins displayed different accumulation patterns in the process of seed development. For example, spot 73 was identified as a protein disulfide isomerase that LOUREIRIN-B continued to accumulate and reached the highest at 20 DAP. Consistent with this in the transcript level, our gene expression analysis also revealed disulfide isomerase can be detected at the late stage of embryogenesis. Plant cysteine proteases are important for organ senescence, plant defense and nutrient mobilization during seed germination, and previous studies reveal cysteine proteinases are up-regulated in various senescing plants, such as Arabidopsis, B. napus, and Nicotiana tabacum. In this study, we identified spot 542 as senescenceassociated cysteine protease, and spot 576 as another cysteine proteinase that increased its abundance all over the five stages, suggesting that cysteine proteinase also played an important role in maturation and senescence of seed growth. Altered accumulation of these proteins indicated active protein Lomitapide Mesylate production and elimination occurred in the process of seed development, which might serve as a monitoring mechanism over those intricate processes of metabolism and energy production. It��s also highly likely that the accumulation of these proteins may be used during rapid cell division and cell structure construction. Despite of these, preponderance of these proteins seemed to be particular of our study, because few of previous reports has indicated so many proteins with similar function, which make us underestimate the importance of protein selfrenewal. Therefore, protein renewal could be an essential regulatory mechanism for seed development. The developing oilseeds take up sugars and amino acids from the surrounding endosome liquid and synthesize large quantities of triacylglycerol storage proteins. Previous work characterizes carbon assimilation during seed filling in Brassica napus and castor, both of which are oil plants. It��s interesting to examine this important metabolism pathway in the seed development. It has been demonstrated that glycolysis supplies most carbon to fatty acid synthesis in rapeseed developing embryos in culture, suggesting glycolysis is essential for carbon assimilation in the developing seeds, but relatively little is known about its regulation and control, and due to the parallel pathways operated in both the cytosol and plastids, it become more complex in plants. Our analysis identified only two storage proteins: napin and cruciferin, which are the two major storage proteins in rape seed, and constitute 20% and 60% of the total protein in mature seeds. As has been reported that their biological synthesis begins early from the expansion phase of embryo development.