tTo increase our understanding of the Dsh DIX domain and its protein partners we conducted a yeast 2-hybrid screen using the N-terminus of Dsh as bait. One Dsh-interacting protein isolated from this screen was the X. laevis ortholog of the transcriptional corepressor, Homeodomain Interacting Protein Kinase-1. Members of the Hipk1 protein family were first identified by virtue of binding to the homeobox genes Nkx1.2, NK-1, NK-3, Nkx2-5, and HoxD4, and subsequently have been reported to also interact with p53, Daxx, and AML1. Hipk1 and the related Hipk2 are together required for neural tube closure, hematopoiesis, angiogenesis and vasculogenesis in mice. Hipk2 but not Hipk1 has previously been implicated in Wnt/b-catenin signaling. No Hipk family member has previously been shown to interact with Dsh, nor to influence b-catenin-independent forms of Wnt signaling. Here, we characterize the functions of Hipk1 during X. laevis development in the context of the Wnt signaling pathways. The Hipk1 ortholog from X. laevis is expressed from the earliest stages of development and in a tissue-specific manner at later stages. Hipk1 binds to Dsh as shown by in vitro binding assays and coimmunoprecipitation from embryo extracts. Hipk1 also binds to the Lef/Tcf family member Tcf3. Hipk1 knock-down in the early embryo leads to a broadening of the expression domains of Wnt/ b-catenin-responsive genes involved in dorsal specification, while knock-down in human tissue culture cells similarly indicates that Hipk1 can repress transcriptional activation of b-catenin-responsive promoter elements. Nevertheless, during gastrulation Hipk1 is necessary for transcriptional activation of a subset of Wntresponsive genes in the involuting mesoderm, and disruption of Hipk1 by either over-expression or knock-down leads to severe defects in convergent extension cell movements. These data suggest that through interactions with both Dsh and Tcf3, Hipk1 acts as an important modulator of Wnt signaling during early vertebrate development. The striking effects of Hipk1 knock-down on some mesoderm markers suggested that 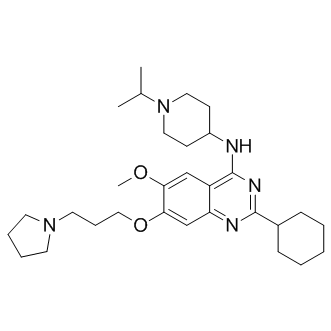 manipulating Hipk1 might also cause severe disruptions in the cell movements that accompany specification of these tissues during gastrulation. To test whether Hipk1 plays a role in regulating these and other cell movements, we performed overexpression and loss-of-function phenotypic experiments in whole embryos. Injection of synthetic RNA encoding Hipk1 into the DMZ resulted in severe gastrulation and neural tube closure defects, demonstrated by a failure to close the blastopore and to fuse the neural tube. The percentage of dorsally-injected Atropine sulfate embryos with the severe gastrulation phenotype was dose-dependent, with higher doses producing more severe effects. In contrast, injection of Hipk1 RNA into the ventral marginal zone resulted in less severely affected embryos with a shortened anterior-posterior length, but a nearly closed blastopore and normal neural tube. One feature of molecules involved in b-catenin-independent pathways, particularly the PCP pathway, is that over-expression Dexrazoxane hydrochloride phenotypes resemble loss-of-function phenotypes at both the cellular and embryonic level. Consistent with a role in such a pathway, phenotypes in Hipk1 morphants closely resembled those observed in embryos over-expressing Hipk1, including comparisons between dorsal versus ventral injections. When either Hipk1MO was injected into the DMZ, phenotypes included shortened embryos with defects in blastopore closure and in neural tube closure.
manipulating Hipk1 might also cause severe disruptions in the cell movements that accompany specification of these tissues during gastrulation. To test whether Hipk1 plays a role in regulating these and other cell movements, we performed overexpression and loss-of-function phenotypic experiments in whole embryos. Injection of synthetic RNA encoding Hipk1 into the DMZ resulted in severe gastrulation and neural tube closure defects, demonstrated by a failure to close the blastopore and to fuse the neural tube. The percentage of dorsally-injected Atropine sulfate embryos with the severe gastrulation phenotype was dose-dependent, with higher doses producing more severe effects. In contrast, injection of Hipk1 RNA into the ventral marginal zone resulted in less severely affected embryos with a shortened anterior-posterior length, but a nearly closed blastopore and normal neural tube. One feature of molecules involved in b-catenin-independent pathways, particularly the PCP pathway, is that over-expression Dexrazoxane hydrochloride phenotypes resemble loss-of-function phenotypes at both the cellular and embryonic level. Consistent with a role in such a pathway, phenotypes in Hipk1 morphants closely resembled those observed in embryos over-expressing Hipk1, including comparisons between dorsal versus ventral injections. When either Hipk1MO was injected into the DMZ, phenotypes included shortened embryos with defects in blastopore closure and in neural tube closure.